Full Tutorial for SigBridgeR
0. Preface
0.1 Contents
-
Full Tutorial for
SigBridgeR
- 0. Preface
- 1. Load and Preprocess data
-
2. Screen
Cells Associated with Phenotype
- 2.1 (Option A) Scissor Screening
- 2.2 (Option B) scPAS Screening
- 2.3 (Option C) scAB Screening
- 2.4 (Option D) scPP Screening
- 2.5 (Option E) DEGAS Screening
- 2.6 (Option F) LP_SGL Screening
- 2.7 (Option G) PIPET Screening
- 2.8 (Option H) SIDISH Screening
- 2.9 (Option I) SCIPAC Screening
- 2.F Merge screening results
- 3. Visualization
- 4. Example
- 5. Troubleshooting
- 6. References
0.2 Introduction to SigBridgeR
SigBridgeR (short for Significant cell-to-phenotype Bridge in R) is an R package for screening cells highly associated with phenotype data using single-cell RNA-seq, bulk RNA expression and sample related phenotype data (e.g. patient survival, age, etc). It integrates many single cell phenotypic screening methods (8. References) and provides unified preprocessing, parameter tuning, and visualization approaches,performing as a unified integration panel.
1. Load and Preprocess data
First load the packages, you will see a version number message indicating successful loading:
library(SigBridgeR)
# ✔ SigBridgeR v3.x.x loaded1.1 Single-cell RNA-seq Data
You can use function SCPreProcess to preprocess your
single-cell RNA-seq data. This function utilizes a flexible pipeline
system, allowing you to customize the analysis workflow using character
codes:
Pipeline Code Table:
| Code | Function | Description |
|---|---|---|
| o | CreateSeuratObject |
Required. Must be the first step. |
| n | NormalizeData |
Standard normalization. |
| s | ScaleData |
Scales data for PCA. |
| v | FindVariableFeatures |
Selects highly variable genes. |
| p | RunPCA |
Principal Component Analysis. |
| e | FindNeighbors |
Computes SNN graph. |
| c | FindClusters |
Louvain algorithm clustering. |
| t | RunTSNE |
t-SNE reduction. |
| u | RunUMAP |
UMAP reduction. |
| r | SCTransform |
SCT workflow. Replaces n, s, v. |
1.1.1 (Option A) Start from Raw Matrix
When starting from a raw count matrix (data.frame, matrix or
dgCMatrix), SCPreProcess executes the steps defined in the
pipeline argument (Default: "onsvpcetu"). You can customize
parameters for each step via the params list.
your_seurat <- SCPreProcess(
sc = your_matrix,
...,
pipeline = "onsvpcetu",
params = list(
# * CreateSeuratObject
o = list(
project = "SC_Screen_Proj",
min.cells = 400L
),
# * NormalizeData
n = list(),
# * ScaleData
s = list(),
# * FindVariableFeatures
v = list(),
# * RunPCA
p = list(),
# * FindNeightbors
e = list(),
# * FindClusters
c = list(
resolution = 0.6
),
# * RunTSNE
t = list(),
# * RunUMAP
u = list()
# * SCTransform
# r = list()
),
quality_control = list(
pattern = c("^MT-")
),
data_filter = list(
nFeature_RNA_thresh = c(200L, 6000L),
nCount_RNA_thresh = c(500L, 50000L),
# * only used when specifed in `quality_control.pattern`
percent.mt = 20L, # mitochondrial genes
percent.rp = 60L # ribosomal protein genes
# ? When combined pattern is used, like `quality_control$pattern <- "^MT-|^RP[LS]"`
# ? Use `_` to separate different patterns like this:
# percent.mt_rp = 60L
# ? When filtering for non-mitochondrial genes and non-ribosomal proteins RNA genes,
# ? the column names are in lowercase letter form with regular expression symbols removed.
# `quality_control$pattern <- "^[rt]rna"`
# Correct threshhold setting is `percent.rt_rna = 60L`
# ? Use `SigBridgeR::Pattern2Colname()` to get the correct colname if still confused.
),
column2only_tumor = NULL
)Key Workflow Steps:
Pipeline Execution: The function runs Seurat methods in the order specified by the
pipelinestring (e.g., ‘o’ then ‘n’).-
QC & Filtering:
Detection: Regex patterns in
quality_control(e.g.,^MT-) automatically generate metadata columns (e.g.,percent.mt).Filtering: Cells are filtered based on
data_filterthresholds. Note: Custom patterns generate specific column names (e.g.,^[rt]rnabecomespercent.rt_rna).
Tumor Filtering: If
column2only_tumoris provided, the function retains only cells with"Tumor/Cancer/Malignant"(case-insensitive) labels in that metadata column.
1.1.2 (Option B) Start from AnnData Object
SCPreProcess natively supports AnnData object. You can use package
anndata or anndataR to read in your AnnData
object from .h5ad file.
First, import the data
reticulate::use_pythonenv("The_path_to_your_python")
# reticulate::use_condaenv("conda_env_name")
anndata_obj <- anndata::read_h5ad("path_to_your_file.h5ad") # Or other file formats, make sure the matrix is in obj$X.Or use anndataR to read in your file:
reticulate::use_pythonenv("The_path_to_your_python")
# reticulate::use_condaenv("conda_env_name")
anndata_obj <- anndataR::read_h5ad("path_to_your_file.h5ad") # basically no difference Then just pass it to SCPreProcess:
your_seurat <- SCPreProcess(
anndata_obj,
pipeline = "onsvpcetu", # Standard pipeline
column2only_tumor = "Tissue" # Optional: keep only tumor cells
)The description of data (meta.data) in anndata_obj$obs
will be add to your_seurat@meta.data.
helpful documentation:
For the structure of anndata, you can refer to
For more quality control, please use scCustmoize,
like scCustomize::Add_Cell_QC_Metrics().
1.1.8 (Optional) Filter Out Tumor Cells
If you already have a Seurat object and aim to filter out
phenotype-associated cells in tumor cells. SCPreProcess
will validate its structure and filter out tumor cells using the
specified metadata column:
your_seurat <- SCPreProcess(sc = your_seurat, column2only_tumor = "Tissue")Note: I don’t recommend using columns like
column2only_tumor = "Celltype"as tumor cell identities vary across tissues. instead:
Create a Dedicated Column: Add a new metadata column (e.g., is_tumor) to explicitly label cells:“Tumo(u)r”/“Normal”
Code Example:
1.2 Bulk expression data
Here are some methods for processing bulk RNA-seq gene expression data matrices.
1.2.1 Evaluate the quality of your bulk RNA-seq data
BulkPreProcess performs comprehensive quality control on
raw bulk RNA-seq count matrix data.
Key parameters for BulkPreProcess:
-
data: Expression matrix with genes as rows and samples as columns, or a list containing count_matrix and sample_info. -
sample_info: Sample information data frame (optional), ignored if data is a list. A qualifiedsample_infoshould contain bothsampleandconditioncolumns (case-sensitive), and there are no specific requirements for the data type stored in theconditioncolumn. -
gene_symbol_conversion: Whether to convert Ensembl version IDs and TCGA version IDs to genes with SymbolConvert in Section 1.2.2, default TRUE. -
check: Whether to perform detailed quality checks, default TRUE. -
min_count_threshold: Minimum count threshold for gene filtering, default 10. -
min_gene_expressed: Minimum number of samples a gene must be expressed in, default 3. -
min_total_reads: Minimum total reads per sample, default 1e6. -
min_genes_detected: Minimum number of genes detected per sample, default 10000. -
min_correlation: Minimum correlation threshold between samples, default 0.8. -
n_top_genes: Number of top variable genes for PCA analysis, default 500. -
show_plot_results: Whether to generate PCA visualization plots, default TRUE. -
verbose: Whether to output detailed information, default TRUE.
Suppose you have bulk RNA-seq count data and sample information, and you want to perform comprehensive preprocessing and quality control. You can refer to and use the following code:
# Example usage of BulkPreProcess
filtered_counts <- BulkPreProcess(
data = your_count_matrix,
sample_info = your_sample_info,
gene_symbol_conversion = TRUE,
check = TRUE,
min_count_threshold = 10,
min_samples_expressed = 3,
min_total_reads = 1e6,
min_genes_detected = 10000,
min_correlation = 0.8,
n_top_genes = 500,
show_plot_results = TRUE,
verbose = TRUE
)The function returns a filtered bulk count matrix after applying quality control steps and does not perform log2 transformation on the data.
Quality Control Metrics Reported
-
Gene Filtering:
First, lowly expressed genes are removed, retaining genes that have an expression level reaching
min_count_thresholdin at leastmin_gene_expressedsamples. This step is based on statistical power considerations, as lowly expressed genes are prone to false-positive results in differential analysis. -
Sample Filtering:
Subsequently, low-quality samples are filtered based on three core indicators:
- Sequencing Depth: The total number of reads must reach
min_total_reads. - Library Complexity: The number of detected genes must exceed
min_genes_detected. - Sample Consistency: When quality checking is enabled, the average
correlation between samples must meet the
min_correlationthreshold.
- Sequencing Depth: The total number of reads must reach
Data Integrity Check: Identifies technical anomalies; too many missing values may indicate experimental issues.
Feature Expression Filtering: Uses a count threshold rather than a proportion threshold to avoid over-penalizing small samples. Genes must be stably expressed in multiple samples to ensure the reliability of statistical tests.
-
Sample-Level Filtering:
Comprehensively evaluates technical quality:
Sequencing depth ensures detection sensitivity, the number of detected genes reflects library diversity, and sample correlation (Pearson) validates experimental reproducibility. PCA analysis further identifies batch effects and outlier samples. -
PCA Variance:
Variance explained by first two principal components.
Whenshow_plot_results = TRUE, the function generates PCA plot colored by experimental condition Batch Effects: Proportion of genes significantly affected by batch
Recommended Parameter Adjustments
Parameter settings need to balance data quality and information retention:
-
For Low-Depth Sequencing Data:
Should relax
min_total_readsandmin_genes_detecteddue to technical limitations rather than quality issues causing low indicators. -
For Noisy Datasets:
Need to increase the
min_correlationthreshold to strengthen sample consistency requirements and exclude technical variation interference. -
For Large Datasets:
Consider setting
check = FALSEto enable only partial filtering functions and speed up the process.
All parameters are set based on empirical values, and users should make appropriate adjustments according to the specific experimental design and sequencing platform characteristics.
1.2.2 Gene Symbol Conversion
SymbolConvert performs a straightforward task:
converting common gene identifiers (e.g., Ensemble IDs, Entrez) to
standardized gene symbols by using the IDConverter
package.
# genes * samples
your_bulk_data <- read.csv("path_to_your_file.csv", header = TRUE, row.names = 1)
your_bulk_data <- SymbolConvert(your_bulk_data)You can also use other package like org.Hs.eg.db for
gene symbol matching if you prefer not to use SymbolConvert’s built-in
IDConverter.
library(org.Hs.eg.db)
your_bulk_data <- read.csv("path_to_your_file.csv", header = TRUE, row.names = 1)
your_bulk_data <- BulkPreProcess(your_bulk_data, gene_symbol_conversion = FALSE)
ensembl_ids <- sub("\\..*", "", rownames(your_bulk_data))
gene_symbols <- mapIds(org.Hs.eg.db,
keys = ensembl_ids,
column = "SYMBOL",
keytype = "ENSEMBL",
multiVals = "first")
rownames(your_bulk_data) <- gene_symbols1.3 Phenotype Data
Basically you can just use your phenotype data directly. If you are
confused about the structure Screen() requires, please
refer to Section 4.
We provide some functions helping formatting and checking your phenotype data.
Checking NA and report the position:
# `max_print`: output how many NA location messages at one time in console if NA exists
CheckNA(your_phenotype_data, max_print = 5L)
mat <- matrix(c(NA,1,1,NA),2, dimnames = list(c("Gene1","Gene2"),c("Sample1","Sample2")))
na_position <- CheckNA(mat)
# ! Found 2 NA values in data
# First 2 positions:
# Row 1 ("Gene1"), col 1 ("Sample1")
# Row 2 ("Gene2"), col 2 ("Sample2")In-place data transformation
Use it just in tidyverse-like style
# ? if a vector
v <- 1:2000
v2 <- PhenoMap(v, v < 1000 ~ "0" ,v > 1000 ~ "1",.default = "here_is_1k")
table(v2)
# 0 1 here_is_1k
# 999 1000 1
v3 <- PhenoMap(v, v < 100 ~ 0, v < 1000 ~ 1, v > 1000 ~ 2, .default = NA_real_)
table(v3)
# 0 1 2
# 99 900 1000
# ? if a data
d <- mtcars
d2 <- PhenoMap(d, mpg > 15 ~ 1, mpg <= 15 ~ 0)
table(d2$mpg)
# 0 1
# 6 26
head(d2)
# mpg cyl disp hp drat wt qsec vs am gear carb
# <num> <num> <num> <num> <num> <num> <num> <num> <num> <num> <num>
# 1: 1 6 160 110 3.90 2.620 16.46 0 1 4 4
# 2: 1 6 160 110 3.90 2.875 17.02 0 1 4 4
# 3: 1 4 108 93 3.85 2.320 18.61 1 1 4 1
# 4: 1 6 258 110 3.08 3.215 19.44 1 0 3 1
# 5: 1 8 360 175 3.15 3.440 17.02 0 0 3 2
# 6: 1 6 225 105 2.76 3.460 20.22 1 0 3 1Direct Interface for Foolproof Data Processing
your_phenotype_data <- PhenoPreProcess(
bulk = your_bulk_data,
phenotype = your_phenotype_data,
phenotype_class = "survival",
status == "death" ~ 1,
status == "alive" ~ 0,
selelct = c("time","status")
)Key parameters for PhenoPreProcess:
-
bulk: Bulk RNA-seq expression data with genes as rows and samples as columns -
phenotype: Phenotype data (Named numeric vector/ data.frame) -
phenotype_class: Type of phenotype data, e.g., “binary”, “survival”, “continuous” -
...: pass toPhenoMap -
select: Columns to select from the phenotype data when it is a data.frame
2. Screen Cells Associated with Phenotype
The function Screen provide 8 different
options for screening cells associated with phenotype, These 8
algorithms come from the repositories mentioned in 6. References, and you can choose one of
them to screen your cells.
Key parameters for Screen:
-
matched_bulk: A data frame of bulk expression data after intersecting samples. Make sure the rownames ofmatched_bulkis identical tophenotype. -
sc_data: A Seurat object after preprocessing, you can use the output ofPreprocessfunction or your own preprocessed Seurat object. -
phenotype: A data frame or named vacor of phenotype data after intersecting samples. See 5. Example for more details. -
label_type: A character value specifying the filtering labels are stored in theSeurat_object@misc. Default:NULL, meaning the name of metho will be used. -
phenotype_class: A character value specifying the phenotype data type, i.e."binary","survival"or"continuous". -
screen_method: A character value specifying the screening method, i.e. “Scissor”, “scPAS”, “scAB”, “scPP”, “DEGAS”, “LP_SGL”, or “PIPET” -
...: Other parameters for the screening methods, see below
2.1 (Option A) Scissor Screening
Parameters pass to ... when using Scissor
method:
-
path2save_scissor_inputs: A character value specifying the path to save intermediate data, you can also setpath2load_scissor_cache = NULLto suppress the saving of intermediate files. Default:Scissor_inputs.RData -
path2load_scissor_cahce: A character value specifying the path to load intermediate data -
alpha: Parameter used to balance the effect of the l1 norm and the network-based penalties. It can be a number or a searching vector. If alpha = NULL, a default searching vector is used. The range of alpha is in[0,1]. A larger alpha lays more emphasis on the l1 norm. -
cutoff: Cutoff for the percentage of the Scissor selected cells in total cells. This parameter is used to restrict the number of the Scissor selected cells. A cutoff less than 50% (default 20%) is recommended depending on the input data. Only used whenalpha = NULL. -
reliability_test: A logical value specifying whether to perform reliability test. Default:FALSE -
reliability_test.n: Permutation times (default: 10) -
reliability_test.nfold: The fold number in cross-validation (default: 10) -
cell_evaluation: A logical value specifying whether to perform cell evaluation. Default:FALSE -
cell_evaluation.benchmark_data: Path to benchmark data (RData file). -
cell_evaluation.FDR: FDR threshold for cell evaluation (default: 0.05). -
cell_evaluation.bootstrap_n: Number of bootstrap iterations for cell evaluation (default: 100).
Usage:
scissor_result = Screen(
matched_bulk = matched_bulk,
sc_data = sc_dataset, # A Seurat object after preprocessing
phenotype = matched_phenotype_data,
label_type = "TP53", # The filtering labels are stored in the `@misc`, you can change it to your own label
phenotype_class = "binary",
screen_method = c("Scissor"),
path2save_scissor_inputs = "Tmp/Scissor_inputs.RData" # Intermediate data
)You can use the intermediate data for repeated runs. This is an
inherent feature of the Scissor.
scissor_result = Screen(
sc_data = sc_dataset,
label_type = "TP53",
phenotype_class = "binary",
screen_method = c("Scissor"),
path2load_scissor_cahe = "Tmp/Scissor_inputs.RData" # Intermediate data
)If only the parameters alpha and cutoff are
adjusted, this method can also be applied.
# When `alpha = NULL`, an alpha iteration will continue until phenotype-associated cells are screened out or no cells are screened out even after exceeding the `cutoff`.
scissor_result = Screen(
sc_data = sc_dataset,
label_type = "TP53",
phenotype_class = "binary",
screen_method = c("Scissor"),
path2load_scissor_cahce = "Tmp/Scissor_inputs.RData", # Intermediate data
alpha = NULL,
cutoff = 0.2
)Returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
scissor_result: The result of Scissor screening, including the parameters used -
reliability_test: Reliability test results -
cell_evaluation: Cell evaluation results
Cell level Evaluation & Reliability Test:
You can use cell_evalutaion = TRUE and
reliability_test = TRUE to obtain some supporting
information for each Scissor selected cell. First, prepare a benchmark
dataset yourself for cell evalutaion.
scissor_result = Screen(
sc_data = sc_dataset,
label_type = "TP53",
phenotype_class = "binary",
screen_method = c("Scissor"),
path2load_scissor_cahce = "Tmp/Scissor_inputs.RData", # Intermediate data
reliability_test = TRUE,
cell_evaluation = TRUE,
cell_evaluation.benchmark_data = "path_to_benchmark_data.RData",
alpha = NULL,
cutoff = 0.05
)helpful documentation:
2.2 (Option B) scPAS Screening
Parameters pass to ... when using scPAS
method (basically adapted from the scPAS’s
documentation):
-
Parameters passed to
scPAS::scPAS()These parameters directly interface with the core
scPAS() function from the original package:-
assay: Name of Assay to get. -
imputation: Logical. imputation or not. -
imputation_method: Character. Name of alternative method for imputation. -
nfeature: Numeric. The Number of features to select as top variable features insc_data. Top variable features will be used to intersect with the features ofmatched_bulk. Default is NULL and all features will be used. -
alpha: Numeric. Parameter used to balance the effect of the l1 norm and the network-based penalties. It can be a number or a searching vector. Ifalpha = NULL, a default searching vector is used. The range of alpha is in[0,1]. A larger alpha lays more emphasis on the l1 norm. -
cutoff: Numeric. Cutoff for the percentage of the scPAS selected cells in total cells whenalpha = NULL. This parameter is used to restrict the number of the scPAS selected cells. A cutoff less than 50% (default 20%) is recommended depending on the input data. -
network_class: The source of feature-feature similarity network. By default this is set to sc and the other one is bulk. -
FDR_threshold: Numeric. FDR value threshold for identifying phenotype-associated cells (default: 0.05) -
independent: Logical. The background distribution of risk scores is constructed independently of each cell. (default: TRUE) -
permutation_times: Number of permutations to perform (default: 2000)
-
usage:
scpas_result = Screen(
matched_bulk = matched_bulk,
sc_data = A_Seurat_object,
phenotype = phenotype,
label_type = "TP53", # The filtering labels are stored in the `@misc`
screen_method = "scPAS",
phenotype_class = "binary",
assay = 'RNA',
imputation = FALSE,
imputation_method = c("KNN", "ALRA"),
nfeature = 3000L,
alpha = c(0.01, NULL),
cutoff = 0.2,
network_class = c("SC", "bulk"),
permutation_times = 2000L,
FDR_threshold = 0.05,
independent = TRUE
)returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
stats: A data.frame the significance of scPAS screening results -
para: A list containing the parameters used in scPAS screening
2.3 (Option C) scAB Screening
Parameters pass to ... when using scAB
method (basically adapted from the scAB’s
documentation):
-
alpha: Coefficient of phenotype regularization, default is0.005. When specifiedNULL, a default searching vector is used. A custom numeric vector is also supported. -
alpha_2: Coefficient of cell-cell similarity regularization, default is0.005. When specifiedNULL, a default searching vector is used. A custom numeric vector is also supported -
maxiter: Maximum number of iterations, default is2000 -
tred: Threshold for early stopping, default is2
usage:
scab_result = Screen(
matched_bulk = your_matched_bulk,
sc_data = A_Seurat_object,
phenotype = your_matched_phenotype,
label_type = "TP53", # The filtering labels are stored in the `@misc`
screen_method = "scAB",
phenotype_class = "binary",
alpha = c(0.005, NULL),
alpha_2 = c(0.005, NULL),
maxiter = 2000L,
tred = 2L
)note:
When both alpha and alpha_2 are specified
as NULL or as search vectors, the total number of searches
equals the product of their lengths, which may lead to long runtimes. In
such cases, we recommend enabling parallel computation.
setFuncOption(parallel = TRUE, parallel.type = 'multisession', workers = 4L)Then simply run the function directly.
returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
scAB_result: A list with the submatrix and loss value
2.4 (Option D) scPP Screening
Parameters pass to ... when using scPP
method :
-
ref_group: The reference group for the binary analysis, default is0 -
Log2FC_cutoff: The cutoff for the log2 fold change of the binary analysis, default is0.585 -
estimate_cutoff: Effect size threshold for continuous traits, default is0.2 -
probs: Quantile cutoff in (0, 0.5) for cell classification, default is0.2. When specifiedNULL, a default searching vector is used. A custom searching vector is also supported.
usage:
# This will take several hours
scpp_result = Screen(
matched_bulk = your_matched_bulk,
sc_data = A_Seurat_object,
phenotype = your_matched_phenotype,
label_type = "TP53", # The filtering labels are stored in the `@misc`
screen_method = "scpp",
phenotype_class = "binary",
ref_group = 0,
Log2FC_cutoff = 0.585,
estimate_cutoff = 0.2,
probs = c(0.2, NULL)
)returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
gene_list: A list containing positive genes and negative genes
2.5 (Option E) DEGAS Screening
Parameters pass to ... when using DEGAS
method
-
sc_data.pheno_colname: The column name of the phenotype in thesc_data@meta.dataslot, used to specify the phenotype for more accurate screening. Default isNULL. -
tmp_dir: The directory for storing the intermediate files. Default isNULL, a directory namedtmpwill be created. -
env_params: A list of parameters for the environment, default islist(). Use?DoDEGASto see the details. -
degas_params: A list of parameters for the DEGAS algorithm, default islist(). Use?DoDEGASto see the details. -
normality_test_method: Method for normality testing:"jarque-bera", "d'agostino", or "kolmogorov-smirnov", default is “jarque-bera”.
degas_result <- Screen(
matched_bulk = your_matched_bulk,
sc_data = A_Seurat_object,
phenotype = your_matched_phenotype,
label_type = "TP53", # The labels are stored in the `@misc` and are used to identify the screening results.
screen_method = "DEGAS",
# The type of phenotype
phenotype_class = c("binary", "continuous", "survival"),
# Environment parameters
env_params = list(
env.name = "r-reticulate-degas",
env.type = "conda",
# Environment.yml file will be used to create the conda environment in default, so other parameters can be ignored
env.method = "environment",
# The path of the environment.yml file
env.file = system.file(
"conda/DEGAS_environment.yml",
package = "SigBridgeR"
),
env.python_verion = "3.9.15",
env.packages = c(
"tensorflow" = "2.4.1",
"protobuf" = "3.20.3"
),
# Force re-creating the env
env.recreate = FALSE,
# Use conda-forge channel for env creating
env.use_conda_forge = TRUE,
# Message output when creating the env
env.verbose = FALSE
),
# DEGAS parameters
degas_params = list(
# DEGAS.model_type will be automatically determined by the `phenotype_class`
DEGAS.model_type = c(
"BlankClass", # only bulk level phenotype specified
"ClassBlank", # only single cell level phenotype specified
"ClassClass", # when both single cell level phenotype and bulk level phenotype specified
"ClassCox", # when both single cell level phenotype and bulk level survival data specified
"BlankCox" # only bulk level survival data specified
),
# DEGAS.architecture will be `DenseNet` in default
DEGAS.architecture = c(
"DenseNet", # a dense net network
"Standard" # a feed forward network
),
# The path to save intermediate data
path.data = '',
path.result = '',
# The python executable path of the conda environment, auto detected in default
DEGAS.pyloc = NULL,
# Some functions will be called in the DEGAS algorithm
DEGAS.toolsPath = paste0(.libPaths()[1], "/DEGAS/tools/"), # or `file.path(.libaPaths()[1], "SigBridgeR/DEGAS_tools/")`
# Screening parameters
DEGAS.ff_depth = 3,
DEGAS.bag_depth = 5,
DEGAS.train_steps = 2000,
DEGAS.scbatch_sz = 200,
DEGAS.patbatch_sz = 50,
DEGAS.hidden_feats = 50,
DEGAS.do_prc = 0.5,
DEGAS.lambda1 = 3.0,
DEGAS.lambda2 = 3.0,
DEGAS.lambda3 = 3.0,
DEGAS.seed = 2
),
# default: "jarque-bera".
normality_test_method = c(
"jarque-bera",
"d'agostino",
"kolmogorov-smirnov"
)
)This code chunk will CREATE a conda environment called
“r-reticulate-degas” with python 3.9.15, and install
the required packages (tensorflow, protobuf) for DEGAS. If an
environment with the same name already exists, it will directly use this
environment without creating a new one (unless specified
env_params = list(env.recreate=TRUE)).
To obtain the default parameters for DEGAS, you can use
SigBridgeR:::DEGASParamSet()
degas_param <- SigBridgeR:::DEGASParamSet(list())
env_param <- SigBridgeR:::DEGASEnvSet(list())You can use ListPyEnvs() to list all the python
environments in your system, including virtual environments. Both
Windows and Unix-like systems are supported. More information can be
found in https://wanglabcsu.github.io/SigBridgeR/articles/Other_Function_Details.html
# * On Unix-like system it goes like this
ListPyEnv()
# name python type
# 1 base /home/user/miniconda3/bin/python conda
# 2 r-reticulate-degas /home/user/miniconda3/envs/r-reticulate-degas/bin/python conda
# 3 test /home/user/.virtualenvs/test/bin/python venvPlease note that the environmental dependencies required for DEGAS to run are quite stringent, and conflicts are highly likely to occur.
returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
model: A model trained using single-cell RNA expression matrix, tissue bulk RNA sequencing expression matrix, and phenotypic data. -
DEGAS_prediction: Using the model to conduct prediction for each cell, resulting in a data.frame where each phenotype has a predicted probability score.
2.6 (Option F) LP_SGL Screening
Parameters pass to ... when using LP_SGL
method
-
resolution: Resolution parameter for Leiden clustering (default:0.6) -
alpha: Alpha parameter for SGL balancing L1 and L2 penalties (default:0.5) -
nfold: Number of folds for cross-validation (default:5) -
dge_analysis: List controlling differential expression analysis:-
run: Whether to run DEG analysis (default:FALSE) -
logFC_threshold: Log fold change threshold (default:1) -
pval_threshold: P-value threshold (default:0.05)
-
lpsgl_result <- Screen(
matched_bulk = your_matched_bulk,
sc_data = A_Seurat_object,
phenotype = your_matched_phenotype,
label_type = "TP53",
resolution = 0.6,
alpha = 0.5,
nfold = 5,
dge_analysis = list(
run = FALSE, # whether to run DEG analysis
logFC_threshold = 1,
pval_threshold = 0.05
),
... # Additional parameters like verbose, seed
)returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
sgl_fit: Fitted SGL model object -
cvfit: Cross-validation results -
dge_res: Differential expression results if requested (NULL otherwise)
2.7 (Option G) PIPET Screening
Parameters pass to ... when using PIPET
method
-
Phenotype adaptation parameters:
-
discretize_method: Character: “kmeans”/“quantile”/“custom” (default: “kmeans”) -
cutoff: Numeric vector for custom discretization whendiscretize_methodis “custom” (default: NULL)
-
-
Marker generation parameters:
-
log2FC: Numeric: log2FC threshold (default: 1) -
p.adjust: Numeric: adjusted p-value threshold (default: 0.05)
-
-
Single-cell annotation parameters:
-
distance: Character: “cosine”/“pearson”/“spearman” (default: “cosine”) -
nPerm: Integer: permutation times for statistical test (default: 1000L)
-
Usage:
pipet_result = Screen(
matched_bulk = matched_bulk,
sc_data = sc_dataset, # A Seurat object after preprocessing
phenotype = matched_phenotype_data,
label_type = "TP53", # The filtering labels are stored in the `Seurat_object@misc`
phenotype_class = "binary", # `survival` is not supported
screen_method = "PIPET",
# PIPET specific parameters
lg2FC = 1,
p.adjust = 0.05,
distance = "cosine",
nPerm = 1000,
parallel = FALSE # Whether to use parallel computing, before using this parameter, please make sure that future::plan() has been called
)Returning structure: A list containing:
-
scRNA_data: A Seurat object after screening (with PIPET annotations in meta.data) -
markers: Phenotype-specific marker genes
2.8 (Option H) SIDISH Screening
Parameters passed to ... when using SIDISH
method:
-
verbose: Logical. Whether to print verbose output during execution. Default isTRUE. -
label_type: Character specifying the phenotype label type stored insc_data@misc. Default is"SIDISH". -
assay: Seurat assay name to use for expression data. Default is"RNA". -
sidish_param: List of parameters for SIDISH algorithm. See below for details. -
env_params: List of parameters for environment setup. See below for details.
sidish_result <- Screen(
matched_bulk = your_matched_bulk,
sc_data = A_Seurat_object,
phenotype = your_survival_phenotype, # Must contain "time" and "status" columns
label_type = "SIDISH", # Labels stored in `@misc` slot to identify screening results
screen_method = "SIDISH",
phenotype_class = "survival", # Currently only survival phenotype is supported
# SIDISH algorithm parameters
sidish_param = list(
# Preprocessing parameters
patient_id = "Sample",
celltype_name = "celltype_major",
processed = TRUE,
n_genes_by_counts = 5000L,
pct_counts_mt = 10L,
batch_correction = FALSE,
survival_ = "time", # Column name for survival time
status = "status", # Column name for event status
# Execution environment
device = "cuda", # "cuda" for GPU acceleration, "cpu" for CPU-only
use_spatial_graph = FALSE,
k_neighbors = NULL,
# Phase 1: VAE training parameters
phase1_epochs = 225L,
phase1_i_epochs = 20L,
phase1_latent_size = 32L,
phase1_layer_dims = c(512L, 128L),
phase1_batch_size = 256L,
phase1_optimizer = "Adam",
phase1_lr = 1e-4,
phase1_lr_3 = 1e-4,
phase1_dropout = 0L,
phase1_type = "Dense", # "Dense" or "Normal"
# Phase 2: Deep Cox training parameters
phase2_epochs = 500L,
phase2_hidden = 128L,
phase2_lr = 1e-4,
phase2_dropout = 0L,
phase2_test_size = 0.2,
phase2_batch_size_bulk = 256L,
# Training & risk definition
train_iterations = 5L,
train_percentile = 0.95,
train_steepness = 30L,
train_path = "./SIDISH_res/", # Path to save intermediate data
train_num_workers = 0L,
train_distribution_fit = "fitted" # "fitted" or "default"
),
# Python environment parameters
env_params = list(
env.name = "r-reticulate-sidish-nvidia", # Auto-selected based on device parameter
env.type = "conda",
env.method = "environment",
env.file = system.file(
"conda/SIDISH_nvidia_environment.yml",
package = "SigBridgeR"
),
env.python_verion = "3.12.12",
env.packages = c(
"numpy" = "1.26.4"
# Additional packages defined in environment.yml
),
env.recreate = FALSE, # Force re-creating environment if TRUE
env.use_conda_forge = TRUE,
env.verbose = FALSE
),
verbose = TRUE
)This code chunk will CREATE a conda environment called
r-reticulate-sidish-nvidia (for GPU) or
r-reticulate-sidish-cpu (for CPU) with
Python 3.12.12 and install all required dependencies for SIDISH. If an
environment with the same name already exists, it will be reused without
recreation (unless env_params = list(env.recreate = TRUE)
is specified).
Note: SIDISH currently only supports
survival phenotypes
(phenotype_class = "survival"). The phenotype data frame
must contain columns named "time" (survival time) and
"status" (event indicator).
To obtain the default parameters for SIDISH, use
SigBridgeR:::SIDISHParamSet() and
SigBridgeR:::SIDISHEnvSet():
# Get default SIDISH algorithm parameters
sidish_default_params <- SigBridgeR:::SIDISHParamSet(list())
# Get default environment parameters (CPU version)
env_default_cpu <- SigBridgeR:::SIDISHEnvSet(list(), device = "cpu")
# Get default environment parameters (GPU version)
env_default_gpu <- SigBridgeR:::SIDISHEnvSet(list(), device = "cuda")You can use ListPyEnvs() to list all Python environments
available on your system (both conda and virtualenv):
ListPyEnv()
# name python type
# 1 base /home/user/miniconda3/bin/python conda
# 2 r-reticulate-sidish-nvidia /home/user/miniconda3/envs/r-reticulate-sidish-nvidia/bin/python conda
# 3 r-reticulate-sidish-cpu /home/user/miniconda3/envs/r-reticulate-sidish-cpu/bin/python condaImportant considerations: - GPU acceleration requires CUDA-compatible hardware. If GPU is not detected but
device = "cuda"is specified, the function will abort with an error message. Setsidish_param = list(device = "cpu")to run on CPU. And of course the python environment must be recreated.
Return structure: A named list containing:
-
scRNA_data: A Seurat object with SIDISH screening results integrated into the@miscand@meta.dataslots under the specifiedlabel_type. The object contains cell-level risk scores and survival-related annotations generated by the SIDISH algorithm.
2.9 (Option I) SCIPAC Screening
Parameters pass to ... when using PIPET
method
-
hvg: Integer. Number of highly variable genes to use for preprocessing. Default is1000L. -
do_pca_sc: Logical. Whether to perform PCA on single-cell data and apply the rotation matrix to bulk data; if FALSE, PCA is performed on bulk data and applied to single-cell data. Default isFALSE. -
n_pc: Integer. Number of principal components to use. Default is60L. -
sc_batch_col: Character or vector. Batch variable for single-cell data. Default isNULL. -
resolution: Integer. Clustering resolution for cell type identification. Default is2L. -
ela_net_alpha: Numeric. Elastic net mixing parameter (0 = ridge, 1 = lasso). Default is0.4. -
bt_size: Integer. Bootstrap sample size for stability assessment. Default is50L. -
ncore: Integer. Number of CPU cores for parallel computation. Default is7L. -
ci_alpha: Numeric. Significance level for confidence intervals. Default is0.05. -
nfold: Integer. Number of folds for cross-validation for regression models. Default is10L. -
...: Additional arguments. Supportsassay(character),verbose(logical), andseed(integer).
scipac_result <- Screen(
matched_bulk = your_matched_bulk,
sc_data = A_Seurat_object,
phenotype = your_matched_phenotype,
label_type = "TP53",
hvg = 1000L,
do_pca_sc = FALSE,
n_pc = 60L,
sc_batch_col = NULL,
resolution = 2L,
ela_net_alpha = 0.4,
bt_size = 50L,
ncore = 7L,
ci_alpha = 0.05,
nfold = 10L,
)returning structure: A list containing:
-
scRNA_data: A Seurat object after screening -
pca_res: PCA rotation results -
cluster_res: Clustering results of single-cell data
2.F Merge screening results
If you have performed multiple screening methods one the same
single-cell data, you can use the MergeResult to merge the
screening results of these methods. The Seurat object or a results list
from Screen is accepted.
merged_seurat = MergeResult(
your_scissor_result,
your_scPAS_result,
your_scAB_result,
your_scPP_result,
your_DEGAS_result
# # * Add more if you want
# ,your_LP_SGL_result,
# your_PIPET_result
)
# * mixed input form is alse supported
merged_seurat = MergeResult(
your_scissor_result$scRNA_data,
your_scPAS_result$scRNA_data,
your_scAB_result,
your_scPP_result,
your_DEGAS_result
# # * Add more if you want
# ,your_LP_SGL_result$scRNA_data,
# your_PIPET_result
)This function merges all slots from the input Seurat objects. Note
that intermediate data (e.g.,
scissor_result$reliability.test or
scab_result$scAB_result) will not be retained during the
merge. While the function can technically combine different single-cell
datasets, it is specifically designed for merging replicates or batches
of the same single-cell RNA-seq experiment; using it for
heterogeneous data may lead to subtle (and potentially hard-to-detect)
errors.
returning structure:
A Seurat object with all merged slots.
3. Visualization
Here we provide some visualization methods for the screening results.
Considering that many people have different needs for data
visualization, SigBridgeR hardly provides visualization
(except for fraction plot and upset plot, because we provide some
statistic results for them). We only provide the source code for
reference.
3.1 UMAP for screening results
example:
Suppose you have performed all algorithm screening on your Seurat object and wish to examine the distribution across different celltypes and patient, you may reference and use the following code:
library(zeallot)
# library(Seurat)
# library(patchwork)
# library(purrr)
c(
celltype_umap,
patient_umap,
scissor_umap,
scab_umap,
scpas_umap,
scpp_umap,
degas_umap,
# * Add more if you want
) %<-%
purrr::map(
# make sure these column names exist
c("celltype", "patient", "scissor", "scAB", "scPAS", "scPP", "DEGAS"),
~ Seurat::DimPlot(
your_seurat_obj,
group.by = .x,
pt.size = 0.05,
reduction = "umap"
) +
ggplot2::ggtitle(.x)
)
# * Show
umaps = celltype_umap +
patient_umap +
scissor_umap +
scab_umap +
scpas_umap +
scpp_umap +
degas_umap +
# * Add more if you want
patchwork::plot_layout(ncol = 3)
umapsThis will generate seven UMAP plots separately.
Or suppose you have performed scPAS screening on your
Seurat object and want to visualize the distribution of prediction
confidence scores, you may reference and use the following code:
library(zeallot)
library(patchwork)
# library(Seurat)
# library(purrr)
c(scPAS_Pvalue_umap, scPAS_NRS_umap) %<-%
purrr::map(
c("scPAS_Pvalue", "scPAS_NRS"),
~ Seurat::FeaturePlot(
object = your_seurat_obj,
features = .x,
) +
ggplot2::ggtitle(.x) +
theme(legend.position = "right")
)
# * Show
scPAS_Pvalue_umap | scPAS_NRS_umapThis will generate two plots, one for each feature specified in
feature.
helpful documentation:
Seurat::DimPlot - https://satijalab.org/seurat/reference/DimPlot.html
Seurat::FeaturePlot - https://satijalab.org/seurat/reference/FeaturePlot.html
3.2 Stack bar plot for screening results
Key parameters for ScreenFractionPlot:
-
seurat_obj: A Seurat object after screening. -
group_by: Used to specify the column of the meta.data inseurat_obj. The plot results will be grouped by this parameter. -
screen_type: Screening algorithm used before. (case-sensitive, e.g., “scissor” for Scissor results) -
show_null: Logical, whether to show groups with zero cells (default: FALSE). -
plot_colorCustom color palette (named vector format):- Required names: “Positive”, “Negative”, “Neutral”, “Other”
- Default: c(“Neutral”=“#CECECE”, “Other”=“#CECECE”, “Positive”=“#ff3333”, “Negative”=“#386c9b”)
Suppose you have already performed the scPAS algorithm
screening on your Seurat object, and you want to check the proportion of
positive cells across different patients. You can refer to and use the
following code:
plot <- ScreenFractionPlot(
screened_seurat = scpas_result$scRNA_data,
group_by = "patient", # grouping basis for the x-axis
screen_type = "scPAS",
plot_title = "scPAS Screening Results"
)If you have performed multiple screening methods and already merged the results, you can use the following code:
plot <- ScreenFractionPlot(
screened_seurat = merged_seurat,
group_by = "patient", # grouping basis for the x-axis
screen_type = c("scPAS", "scAB", "scPP"), # multiple screening results
plot_title = "Screening Results" # A screen_type prefix will be added to the current plot title
)The order of the groups is determined by the proportion of Positive cells within each group.
returning structure:
If a single screen_type is specified
-
stats: A data frame containing the proportion of positive cells for each group. -
plot: A ggplot2 object.
If multiple screen_types are specified
-
stats: A list containing data frames containing the proportion of positive cells for each group. -
plot: A list containing each ggplot2 objects. -
combined_plot: A ggplot2 object containing all the plots (2*2 grid).
3.3 Venn diagram for screening results
ggVennDiagram is used to generate a Venn diagram for the
screening results. Suppose you have performed some of the screening
algorithms on your Seurat object, and you want to check the overlap of
the cells selected by each algorithm. You can refer to and use the
following code:
example:
library(ggVennDiagram)
# # * If you have merged the results, you can use the following code instead:
# c(scissor_pos, scab_pos, scpas_pos, scpp_pos, degas_pos) %<-%
# purrr::map(
# c("scissor", "scAB", "scPAS", "scPP", "DEGAS"),
# ~ colnames(merged_seurat)[
# which(merged_seurat[[.x]] == "Positive")
# ]
# )
# * get the cell vectors
scissor_pos <- colnames(scissor_result$scRNA_data)[
which(scissor_result$scRNA_data$scissor == "Positive")
]
scab_pos <- colnames(scab_result$scRNA_data)[
which(scab_result$scRNA_data$scAB == "Positive")
]
scpas_pos <- colnames(scpas_result$scRNA_data)[
which(scpas_result$scRNA_data$scPAS == "Positive")
]
scpp_pos <- colnames(scissor_result$scRNA_data)[
which(scpp_result$scRNA_data$scPP == "Positive")
]
degas_pos <- colnames(degas_result$scRNA_data)[
which(degas_result$scRNA_data$DEGAS == "Positive")
]
all_cells <- colnames(your_seurat_obj)
# * create a list of cell vectors
pos_venn = list(
scissor = scissor_pos,
scpas = scpas_pos,
scab = scab_pos,
scpp = scpp_pos,
degas = degas_pos,
all_cells = all_cells
# * you can add more groups here
)
set.seed(123)
venn_plot = ggVennDiagram::ggVennDiagram(
x = pos_venn,
# * the labels of each group to be shown on the diagram
category.names = c(
"Scissor",
"scPAS",
"scAB",
"scPP",
"DEGAS",
"All cells"
),
# * the colors of each group
set_color = c(
"#a33333ff",
"#37ae00ff",
"#2a2a94ff",
"#9c8200ff",
"#bb14adff",
"#008383ff"
)
) +
ggplot2::scale_fill_gradient(low = "white", high = "#ffb6b6ff") +
ggplot2::ggtitle("Screening Venn Diagram")helpful link:
3.4 Upset plot for screening results
If too many screening meyhods are selected, the number of intersections among cells screened by different methods will also increase. In this case, using an upset plot is more intuitive and neat than a Venn diagram.
ggupset is used for visualizing upset plot.
Key parameters for ScreenUpset:
-
screened_seurat: A Seurat object after screening. -
screen_type: Screening algorithm used before. (case-sensitive, e.g., “scissor” for Scissor results) -
n_intersections: Number of intersections to display in the plot. Default: 20. -
x_lab: Label for the x-axis. Default: “Screen Set Intersections”. -
y_lab: Label for the y-axis. Default: “Number of Cells”. -
title: Plot title. Default: “Cell Counts Across Screen Set Intersections”. -
bar_color: Color for the bars in the plot. Default: “#4E79A7”. -
combmatrix_point_color: Color for points in the combination matrix. Default: “black”. -
...: Additional arguments passed toggplot2::theme()for customizing the plot appearance.
upset <- ScreenUpset(screened_seurat = merged_seurat)returning structure:
-
plot: A ggplot2 object. -
stats: A data frame containing the numbers of positive cells for each group.
helpful link:
ggupset - https://github.com/const-ae/ggupset
4. Example
4.1 Survival-associated cell screening
Here we use the example data LUAD to demonstrate how to use the
functions in SigBridgeR to screen cells associated with
phenotype.
# Set working directory
if (requireNamespace("here", quietly = TRUE)) {
here::i_am(".here")
setwd(here::here())
knitr::opts_knit$set(root.dir = here::here())
}
library(SigBridgeR)
library(zeallot)
# * load the example data
c(mat_exam, bulk, pheno) %<-% LoadRefData(data_type = "survival")
dim(mat_exam)
#[1] 33694 1093
mat_exam[1:6, 1:2]
# SMC01.T_AAACCTGCATACGCCG SMC01.T_AAACCTGGTCGCATAT
# A1BG 0 0
# A1BG.AS1 0 0
# A1CF 0 2
# A2M 0 0
# A2M.AS1 0 0
# A2ML1 0 0
dim(bulk)
# [1] 4071 506
bulk[1:6,1:6] # already log2 transformed
# TCGA-69-7978 TCGA-62-8399 TCGA-78-7539 TCGA-73-4658 TCGA-44-6775 TCGA-44-2655
# HIF3A 4.2598 11.6239 9.1362 5.0288 4.0573 5.5335
# RTN4RL2 8.2023 5.5819 3.5365 7.4156 7.7107 5.3257
# HMGCLL1 2.7476 5.8513 3.8334 3.6447 2.9188 4.8820
# LRRTM1 0.0000 0.4628 4.7506 6.8005 7.7819 2.2882
# GRIN1 6.6074 5.4257 4.9563 7.3510 3.5361 3.3311
# LRRTM3 1.7458 2.0092 0.0000 1.4468 0.0000 0.0000
nrow(pheno)
# [1] 506
head(pheno)
# time status
# TCGA-69-7978 4.40 0
# TCGA-62-8399 88.57 0
# TCGA-78-7539 25.99 0
# TCGA-73-4658 52.56 1
# TCGA-44-6775 23.16 0
# TCGA-44-2655 43.50 0This single-cell RNA data is from humans. We set many parameters to
NULL or 0 in order to maximize the flexibility
of downstream analyses and capture a broader range of biological
signals, so as to avoid insignificant results caused by too small a
dataset.
In practical data analysis, please adjust the thresholds and parameters as needed for your specific use case.
Now we use SCPreProcess() to pre-process the single-cell
RNA expression matrix data.
seurat <- SCPreProcess(
sc = mat_exam,
params = list(
o = list(
min.cells = 0,
min.features = 0
),
s = list(
features = rownames(mat_exam)
)
),
quality_control = list(),
data_filter = list()
)BulkPreProcess() is to pre-process the bulk expression
data and the related clinical data.frame (containing bulk sample
information) to perform PCA. Here, we want to retain the data so that
the screening can reflect the most accurate situation. You can choose
whether to filter or retain based on your own needs. See also 1.2 Bulk expression data for more
details.
To facilitate the demonstration of the use of the
sample_info parameter, we will divide the
pheno based on its survival status into two groups, which
will be stored in the condition column. batch
column is used to store the batch information of the samples. The
example is from one single batch.
In practice, please replace this with your actual phenotype classification; the approach shown here is for demonstration purposes only and is not recommended for real analyses.
sample_info <- tibble::rownames_to_column(pheno, var = "sample") %>%
dplyr::rename(condition = status)
sample_info$batch <- "batch1"
head(sample_info)
# sample time condition batch
# 1 TCGA-69-7978 4.40 0 batch1
# 2 TCGA-62-8399 88.57 0 batch1
# 3 TCGA-78-7539 25.99 0 batch1
# 4 TCGA-73-4658 52.56 1 batch1
# 5 TCGA-44-6775 23.16 0 batch1
# 6 TCGA-44-2655 43.50 0 batch1
set.seed(123)
# * generate a mock data
gene_ids <- c(
paste0("ENSG", sprintf("%011d", 1:3900)),
paste0("GENE_", LETTERS[1:100])
)
library(MASS)
mu <- c(
runif(500, 100, 2000), # high
runif(1500, 20, 200), # medium
rexp(2000, rate = 1 / 2) # low (gamma-like, many near-zero)
)
lib_size_factor <- rlnorm(506, meanlog = log(3e7), sdlog = 0.3) / 3e7 # ~20–60M reads
counts_mat <- matrix(0L, nrow = 4000, ncol = 506)
for (i in seq_len(4000)) {
mu_i <- mu[i] * lib_size_factor
# dispersion decreases with mean: phi ≈ 0.5 / sqrt(mu_i + 1)
phi_i <- pmax(0.05, 0.5 / sqrt(mu_i + 1))
size_i <- 1 / phi_i
counts_mat[i, ] <- rnbinom(506, mu = mu_i, size = size_i)
}
counts_mat <- floor(counts_mat)
counts_mat[counts_mat < 0] <- 0L
colnames(counts_mat) <- sample_info$sample
rownames(counts_mat) <- gene_ids
filtered_counts_mat <- BulkPreProcess(
data = counts_mat,
sample_info = sample_info,
min_count_threshold = 10L,
min_gene_expressed = 3L,
min_total_reads = 1e5L,
min_genes_detected = 1000L,
min_correlation = 0.8,
n_top_genes = 500L, # used in pca
show_plot_results = TRUE
)
# ✔ [2025/12/23 15:19:20] Data loaded: 4000 genes * 506 samples
# ℹ Aggregate Duplicated genes in rownames
# ℹ [2025/12/23 15:19:21] Starting detailed quality checks...
# ✔ Sample correlation: Good (minimum = 0.95)
# ℹ [2025/12/23 15:19:23] PCA completed: PC1(0.99%) PC2(0.83%), 27 outlier samples
# Warning: Detected 27 outlier sample(s) : TCGA-86-A456, TCGA-55-A492, TCGA-78-8660, TCGA-69-7973, TCGA-69-7980, TCGA-97-7937, TCGA-55-6543, TCGA-86-8671, TCGA-86-8359, TCGA-05-4405, TCGA-62-A471,
# TCGA-35-3615, TCGA-05-5428, TCGA-99-8025, TCGA-44-6779, TCGA-4B-A93V, TCGA-MP-A4T7, TCGA-55-8203, …, TCGA-55-8301, and TCGA-55-7727
# ℹ [2025/12/23 15:19:23] Generating visualization plots...
# ✔ [2025/12/23 15:19:24] Data filtering completed:
# ℹ Genes: 4000 -> 2703 (removed 1297)
# ℹ Samples: 506 -> 506 (removed 0)
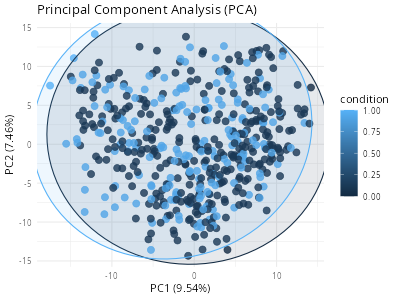
# ✔ [2025/12/23 15:19:24] BulkPreProcess completedAnd we will see a PCA plot.
knitr::include_graphics("vignettes/example_figures/bulk_preprocess_pca.png")Thus far, we have completed all the data preprocessing. We are now ready to formally employ various single-cell phenotypic screening algorithms.
First, we use scissor to screen cells associated with
survival.
scissor_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "survival",
phenotype_class = "survival",
screen_method = "Scissor",
alpha = 0.05
)
# ℹ [2025/09/08 17:03:20] Scissor start...
# ℹ [2025/09/08 17:03:20] Start from raw data...
# ℹ Using "RNA_snn" graph for network.
# ℹ [2025/09/08 17:03:20] Normalizing quantiles of data...
# ℹ [2025/09/08 17:03:20] Subsetting data...
# ℹ [2025/09/08 17:03:21] Calculating correlation...
# ----------------------------------------------------------------------------------------------------
# Five-number summary of correlations:
# 0% 25% 50% 75% 100%
# -0.2342323 0.0594385 0.1118708 0.1642065 0.5250605
# ----------------------------------------------------------------------------------------------------
# ℹ [2025/09/08 17:03:21] Perform cox regression on the given clinical outcomes:
# ✔ Statistics data saved to `Scissor_inputs.RData`.
# ℹ [2025/09/08 17:03:22] Screening...
#
# ── At alpha = 0.05 ──
#
# Scissor identified 265 Scissor+ cells and 73 Scissor- cells.
# The percentage of selected cell is: 30.924%
table(scissor_result$scRNA_data$scissor)
# Negative Neutral Positive
# 73 755 265 You will see an additional “Scissor_inputs.RData” in the working
directory. This is the intermediate data generated by the Scissor
method, which we can use to save running time ( You can also set
path2load_scissor_cache=NULL to suppress the saving of
intermediate files ). Meanwhile, we set
reliability_test=TRUE, which will run an additional
reliability test.
scissor_result <- Screen(
matched_bulk = bulk, # doesn't need to be provided Since the intermediate data is provided
sc_data = seurat,
phenotype = pheno_ok, # doesn't need to be provided Since the intermediate data is provided
label_type = "survival",
phenotype_class = "survival",
screen_method = "Scissor",
alpha = 0.05,
path2load_scissor_cache = "Scissor_inputs.RData",
reliability_test = TRUE
)
# ℹ [2025/09/08 16:07:48] Scissor start...
# ℹ [2025/09/08 16:07:48] Loading data from `Scissor_inputs.RData`...
# ℹ [2025/09/08 16:07:48] Screening...
# [1] "alpha = 0.05"
# [1] "Scissor identified 249 Scissor+ cells and 245 Scissor- cells."
# [1] "The percentage of selected cell is: 45.197%"
# --------------------------------------------------------------------------------
# ℹ [2025/09/08 16:07:54] Start reliability test
# Attaching package: ‘survival’
# The following object is masked from ‘package:future’:
# cluster
# [1] "|**************************************************|"
# [1] "Perform cross-validation on X with true label"
# Finished!
# [1] "|**************************************************|"
# [1] "Perform cross-validation on X with permutated label"
# Finished!
# [1] "Test statistic = 0.590"
# [1] "Reliability significance test p = 0.000"
# ✔ [2025/09/08 16:10:54] reliability test: Done
scissor_result$reliability_result$statistic
# [1] 0.5899587
scissor_result$reliability_result$p
# [1] 0
scissor_result$reliability_result$c_index_test_real
# [1] 0.6038544 0.5022222 0.6050725 0.6279391 0.5064935 0.6033520 0.6769231 0.6453089 0.4968421 0.6315789
scissor_result$reliability_result$c_index_test_back %>% unlist() %>% matrix(nrow = 10)
# [,1] [,2] [,3] [,4] [,5] [,6] [,7] [,8] [,9] [,10]
# [1,] 0.5594714 0.4943820 0.5379747 0.5583658 0.5527728 0.6146435 0.5130597 0.5701275 0.4691943 0.5177305
# [2,] 0.5735608 0.4726891 0.4146341 0.5020661 0.5354331 0.6840149 0.5540275 0.4990758 0.5324484 0.5497382
# [3,] 0.5960145 0.5157116 0.6191446 0.5091912 0.5659656 0.5870370 0.5090580 0.4247788 0.5734266 0.5539715
# [4,] 0.6210191 0.6563147 0.5581948 0.4675615 0.4688222 0.5308219 0.5100402 0.6709091 0.6155779 0.5607143
# [5,] 0.4623656 0.5716695 0.4920441 0.5403727 0.5555556 0.5911215 0.5738499 0.6189258 0.5871560 0.6084337
# [6,] 0.5868794 0.5127660 0.7416880 0.5366876 0.5417440 0.5633803 0.5161943 0.4830372 0.4732965 0.5740132
# [7,] 0.4975610 0.5751503 0.4801902 0.5588972 0.4940898 0.5723370 0.5788382 0.6171875 0.5884413 0.5445545
# [8,] 0.6331361 0.5970516 0.5473888 0.6274131 0.5159705 0.5000000 0.5299760 0.4905660 0.5277778 0.5027322
# [9,] 0.6390805 0.6548913 0.5049310 0.6299639 0.6385135 0.5964392 0.5381605 0.7462687 0.5185185 0.6585859
# [10,] 0.5207373 0.5000000 0.6051780 0.4932127 0.6892430 0.4786517 0.5619266 0.6614583 0.6502242 0.5333333Next, we use scPAS, scAB, scPP, LP_SGL and PIPET to do the same
screening. Generally you only need to change the
screen_method, as long as you have not specified any
particular parameters.
scpas_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "survival",
phenotype_class = "survival",
screen_method = "scPAS",
alpha = 0.05
)
# ℹ [2025/10/20 16:24:27] Start scPAS screening.
# ℹ [2025/10/20 16:24:29] Quantile normalization of bulk data.
# ℹ [2025/10/20 16:24:29] Extracting single-cell expression profiles...
# ℹ [2025/10/20 16:24:29] Constructing a gene-gene similarity by single cell data...
# Building SNN based on a provided distance matrix
# Computing SNN
# ℹ [2025/10/20 16:24:30] Optimizing the network-regularized sparse regression model...
# ℹ [2025/10/20 16:24:30] Perform cox regression on the given phenotypes...
#
# ── At alpha = 0.05 ──
#
# lambda = 0.776168989003421
# scPAS identified 59 risk+ features and 61 risk- features.
# The percentage of selected feature is: 13.73%
# ℹ [2025/10/20 16:24:49] Calculating quantified risk scores...
# ℹ [2025/10/20 16:24:49] Qualitative identification by permutation test program with 2000 times random perturbations...
# ✔ [2025/10/20 16:24:50] scPAS screening done.
table(scpas_result$scRNA_data$scPAS)
# Negative Neutral Positive
# 5 1085 3 As you can see, due to differences in data and algorithms, not every
screening algorithm is able to screen out cells. You can adjust the
corresponding parameters, e.g. change the alpha to
NULL, this will make scPAS iterate alpha until the result
is significant (or judged as having no significant cell subpopulations).
See also 3.2 (Option B) scPAS
Screening
scpas_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "survival",
phenotype_class = "survival",
screen_method = "scPAS",
alpha = NULL,
cutoff = 0.2
)
# ℹ [2025/10/20 16:43:31] Start scPAS screening.
# ℹ [2025/10/20 16:43:32] Quantile normalization of bulk data.
# ℹ [2025/10/20 16:43:32] Extracting single-cell expression profiles...
# ℹ [2025/10/20 16:43:32] Constructing a gene-gene similarity by single cell data...
# Building SNN based on a provided distance matrix
# Computing SNN
# ℹ [2025/10/20 16:43:33] Optimizing the network-regularized sparse regression model...
# ℹ [2025/10/20 16:43:33] Perform cox regression on the given phenotypes...
#
# ── At alpha = 0.001 ──
#
# lambda = 7.61825638357188
# scPAS identified 315 risk+ features and 366 risk- features.
# The percentage of selected feature is: 77.918%
#
# ── At alpha = 0.005 ──
#
# lambda = 3.68743152046651
# scPAS identified 193 risk+ features and 231 risk- features.
# The percentage of selected feature is: 48.513%
#
# ── At alpha = 0.01 ──
#
# lambda = 2.55333682907113
# scPAS identified 137 risk+ features and 158 risk- features.
# The percentage of selected feature is: 33.753%
#
# ── At alpha = 0.05 ──
#
# lambda = 0.776168989003421
# scPAS identified 59 risk+ features and 61 risk- features.
# The percentage of selected feature is: 13.73%
# ℹ [2025/10/20 16:44:55] Calculating quantified risk scores...
# ℹ [2025/10/20 16:44:55] Qualitative identification by permutation test program with 2000 times random perturbations...
# ✔ [2025/10/20 16:44:57] scPAS screening done.Generally speaking, a larger alpha means looser screening. However,
it still won’t result in the occurence of too many false-positive cells.
You can also adjust the cutoff parameter as you like.
table(scpas_result$scRNA_data$scPAS)
# Negative Neutral Positive
# 5 1085 3 Now we use scAB, scPP, DEGAS, LP_SGL and PIPET to screen cells.
scab_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "survival",
phenotype_class = "survival",
screen_method = "scAB"
)
# ℹ [2025/09/08 17:04:51] Start scAB screening.
# ℹ Using "RNA_snn" graph for network.
# ℹ [2025/09/08 17:04:52] Selecting K...
# ℹ [2025/09/08 17:06:15] Run NMF with phenotype and cell-cell similarity regularization at K = 3.
# ℹ [2025/09/08 17:06:19] Screening cells...
# ℹ [2025/09/08 17:06:19] scAB screening done.
table(scab_result$scRNA_data$scAB)
# Other Positive
# 1018 75
scpp_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "survival",
phenotype_class = "survival",
screen_method = "scPP"
)
# ℹ [2025/09/08 17:00:28] Start scPP screening.
# ℹ [2025/09/08 17:00:28] Finding markers...
# Warning in coxph.fit(X, Y, istrat, offset, init, control, weights = weights, :
# Loglik converged before variable 1 ; coefficient may be infinite.
# ℹ [2025/09/08 17:00:52] Screening...
# Genes in the gene sets NOT available in the dataset:
# gene_pos: 13 (6% of 230)
# gene_neg: 54 (12% of 446)
# There are no genes significantly upregulated in Phenotype- compared to Phenotype+.
# ✔ [2025/09/08 17:00:54] scPP screening done.
table(scpp_result$scRNA_data$scPP)
# Negative Neutral Positive
# 52 993 48
# I recommend running this code in the background.
degas_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "This_is_a_DEGAS_test",
phenotype_class = "survival",
screen_method = "DEGAS"
)
# ℹ [2025/10/09 18:41:00] Starting DEGAS Screen
# ℹ [2025/10/09 18:41:01] Setting up Environment...
# ℹ [2025/10/09 18:41:08] Training DEGAS model...
# ℹ [2025/10/09 18:41:08] 3-layer DenseNet BlankCox DEGAS model
# ℹ [2025/10/09 18:41:10] Python check passed, using Python 3.9.15
# ℹ [2025/10/09 18:41:10] Training...
###########################
# Many output from python #
###########################
# ℹ [2025/10/10 17:35:04] Predicting and Labeling...
# ℹ [2025/10/10 17:35:04] Labeling screened cells...
# ℹ [2025/10/10 17:35:04] Searching for survival-associated cells...
# ℹ Scores over 0.499 are considered `Positive`.
# ℹ [2025/10/10 17:35:04] DEGAS Screen done.
table(degas_result$scRNA_data$DEGAS)
# Other Positive
# 1038 55
# I recommend running this code in the background.
lpsgl_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
label_type = "LP_SGL",
phenotype_class = "survival",
screen_method = "LP_SGL"
)
# ℹ [2025/11/22 21:14:29] Starting LP-SGL screening analysis
# ℹ [2025/11/22 21:14:29] Fetch graph from Seurat object
# ℹ [2025/11/22 21:14:29] Run Leiden clustering
# ℹ [2025/11/22 21:14:30] Calculating correlation matrix...
# ℹ [2025/11/22 21:14:33] Fitting SGL model with alpha = 0.5, this may take a while
# ℹ [2025/11/22 21:14:49] Running 5-fold cross-validation
# ℹ [2025/11/22 21:17:31] Optimal lambda index: 20 (error = 1380.7537932881)
# ℹ [2025/11/22 21:17:31] LP-SGL screening completed
table(lpsgl_result$scRNA_data$LP_SGL)
# Negative Neutral Positive
# 77 778 233 Note that PIPET does not support survival phenotype. We will show how to use PIPET in Section 5.3
After these algorithms have been run, the four sets of data can be merged since screening methods performed on the same data.
screen_result <- MergeResult(
scissor_result,
scpas_result,
scab_result,
scpp_result,
degas_result,
lpsgl_result
)
# ✔ Successfully merged 6 objects.
class(screen_result)
# [1] "Seurat"
# attr(,"package")
# [1] "SeuratObject"
colnames(screen_result[[]])
# [1] "orig.ident" "nCount_RNA" "nFeature_RNA" "test_col" "percent.rp" "percent.mt" "RNA_snn_res.0.1" "seurat_clusters"
# [9] "scissor" "scAB" "scAB_Subset1" "Subset1_loading" "scAB_Subset2" "Subset2_loading" "scPAS_RS" "scPAS_NRS"
# [17] "scPAS_Pvalue" "scPAS_FDR" "scPAS" "scPP_AUCup" "scPP_AUCdown" "scPP" "DEGAS" "LP_SGL" Finally, we can visualize the screening results. Let’s start with a Venn diagram to see the situation.
library(ggVennDiagram)
library(zeallot)
# * color palette
set.seed(123)
my_colors <- randomcoloR::distinctColorPalette(
length(unique(screen_result$seurat_clusters)),
runTsne = TRUE
)
c(scissor_pos, scab_pos, scpas_pos, scpp_pos, degas_pos, lpsgl_pos) %<-%
purrr::map(
c("scissor", "scAB", "scPAS", "scPP", "DEGAS", "LP_SGL"),
~ colnames(screen_result)[
which(screen_result[[.x]] == "Positive")
]
)
all_cells <- colnames(screen_result)
# * create a list of cell vectors
pos_venn <- list(
scissor = scissor_pos,
scpas = scpas_pos,
scab = scab_pos,
scpp = scpp_pos,
degas = degas_pos,
lp_sgl = lpsgl_pos,
all_cells = all_cells
)
venn <- ggVennDiagram::ggVennDiagram(
x = pos_venn,
# * the labels of each group to be shown on the diagram
category.names = c(
"Scissor",
"scPAS",
"scAB",
"scPP",
"DEGAS",
"LP_SGL",
"All cells"
),
# * the colors of each group
set_color = c(
"#a33333ff",
"#37ae00ff",
"#0000f5ff",
"#d4b100ff",
"#e600eeff",
"#59108aff",
"#008383ff"
),
label_geom = "text"
) +
ggplot2::scale_fill_gradient(low = "white", high = "#ffb6b6ff") +
ggplot2::ggtitle("Screening Venn Diagram")
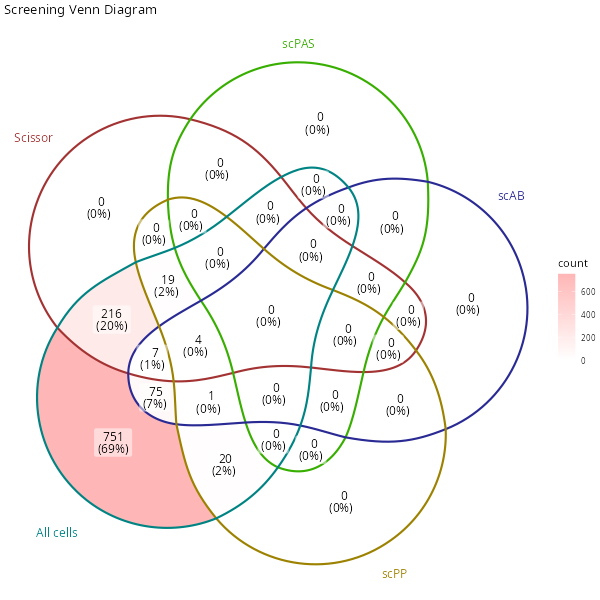
venn
# ggplot2::ggsave(
# "vignettes/example_figures/venn.png",
# plot = venn,
# width = 10,
# height = 10
# )
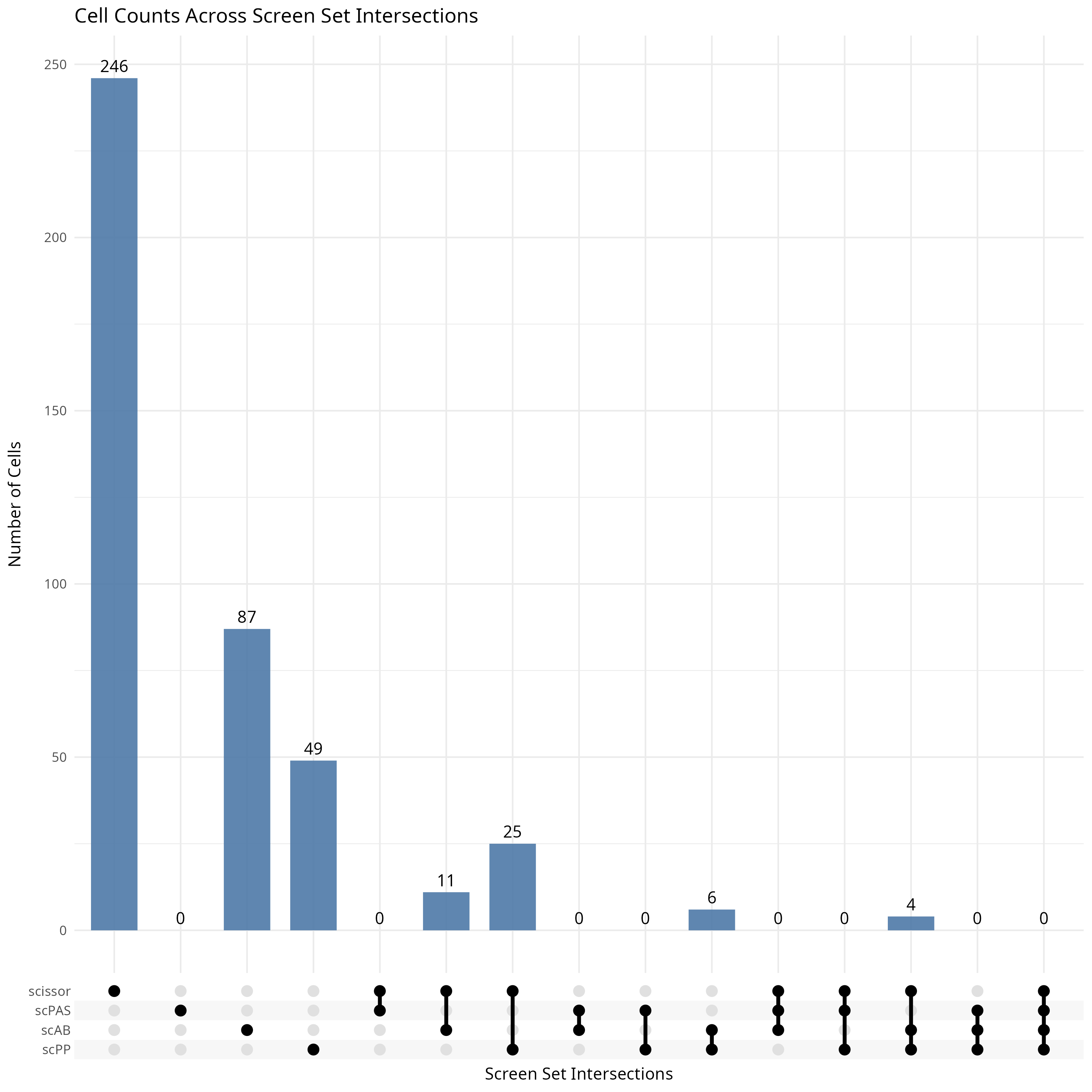
knitr::include_graphics("vignettes/example_figures/venn.png")When there are too many sets, the visualization effect of the Venn diagram is not very good. We can use a set plot instead. Only positive cells are shown in the set plot.
upset <- ScreenUpset(
screened_seurat = screen_result,
screen_type = c("scissor", "scPAS", "scAB", "scPP", "DEGAS","LP_SGL"),
n_intersections = 40
)
# * show the cell numbers of each set
head(upset$stats)
# # A tibble: 6 × 3
# intersection sets count
# <chr> <named list> <dbl>
# 1 scissor <chr [1]> 265
# 2 scPAS <chr [1]> 2
# 3 scAB <chr [1]> 75
# 4 scPP <chr [1]> 49
# 5 DEGAS <chr [1]> 55
# 6 LP_SGL <chr [1]> 233
# ggplot2::ggsave(
# "vignettes/example_figures/upset.png",
# plot = upset$plot,
# width = 10,
# height = 10
# )
knitr::include_graphics("vignettes/example_figures/upset.png")A bar chart showing proportions can also be used to examine the
screening results. Since the example data does not have sample metadata,
we have created a fictional Sample column.
set.seed(123)
# * fictional sample column
screen_result$Sample <- sample(
paste0("Sample", 1:10),
ncol(screen_result),
replace = TRUE
)
table(screen_result$Sample)
# Sample1 Sample10 Sample2 Sample3 Sample4 Sample5 Sample6 Sample7 Sample8 Sample9
# 98 117 96 119 95 100 101 131 115 121
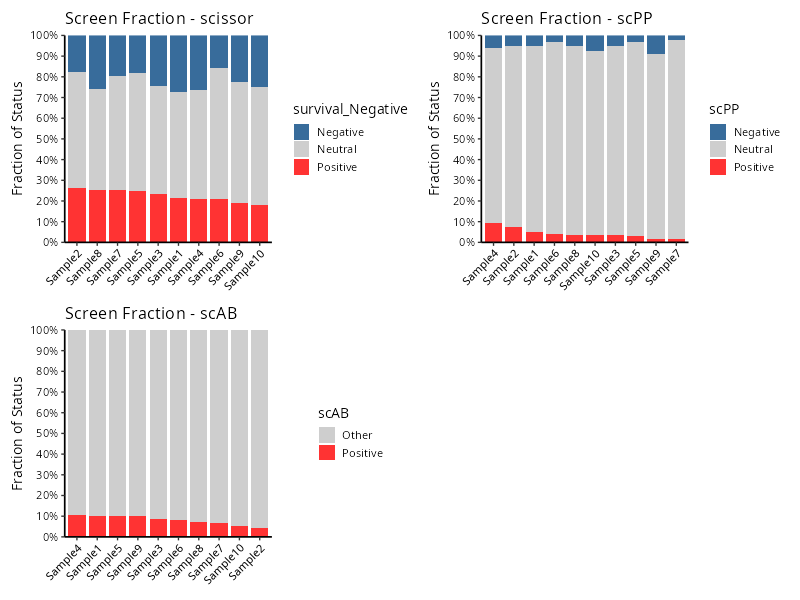
fraction_list <- ScreenFractionPlot(
screened_seurat = screen_result,
group_by = "Sample",
screen_type = c("scissor", "scPP", "scAB", "scPAS", "DEGAS", "LP_SGL"),
show_null = FALSE,
plot_color = NULL,
show_plot = TRUE
)
# Creating plots for 6 screen types...
# ggplot2::ggsave(
# "vignettes/example_figures/fraction.png",
# plot = fraction_list$combined_plot,
# width = 10,
# height = 10
# )
knitr::include_graphics("vignettes/example_figures/fraction.png")As you see, the label_types set in function
Screen are shown in the legend of each plot.
The fraction_list contains the statistical data and
charts for each screening algorithm. The title of each plot will be
appended with the method name as an identifier.
fraction_list
├── stats
│ ├── scissor
│ ├── scAB
│ ├── scPAS
│ ├── scPP
│ ├── DEGAS
│ └── LP_SGL
├── plots
│ ├── scissor
│ ├── scAB
│ ├── scPAS
│ ├── scPP
│ ├── DEGAS
│ └── LP_SGL
└── combined_plot # show 6 plots in one plot
UMAP is the most commonly used type of plot in academic literature.
library(patchwork)
library(zeallot)
my_palette <- randomcoloR::distinctColorPalette(
k = length(unique(screen_result$Sample)),
runTsne = TRUE
)
sample_umap <- Seurat::DimPlot(
screen_result,
group.by = "Sample",
pt.size = 1.2,
alpha = 0.8,
reduction = "umap",
cols = my_palette
) +
ggplot2::ggtitle("Sample")
c(
scissor_umap,
scab_umap,
scpas_umap,
scpp_umap,
degas_umap,
lpsgl_umap
) %<-%
purrr::map(
c("scissor", "scAB", "scPAS", "scPP", "DEGAS", "LP_SGL"), # make sure these column names exist
~ Seurat::DimPlot(
screen_result,
group.by = .x,
pt.size = 1.2,
alpha = 0.8,
reduction = "umap",
cols = c(
"Neutral" = "#CECECE",
"Other" = "#CECECE",
"Positive" = "#ff3333",
"Negative" = "#386c9b"
)
) +
ggplot2::ggtitle(.x)
)
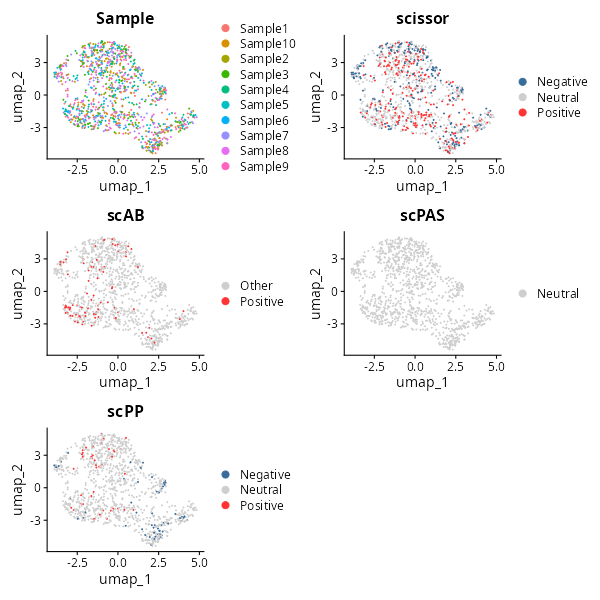
# * Show
umaps <- sample_umap +
scissor_umap +
scab_umap +
scpas_umap +
scpp_umap +
degas_umap +
lpsgl_umap +
patchwork::plot_layout(ncol = 2)
umaps
# ggplot2::ggsave(
# "vignettes/example_figures/umaps.png",
# plot = umaps,
# width = 10,
# height = 10
# )
knitr::include_graphics("vignettes/example_figures/umaps.png")All the analysis has been completed, and we can save the data for future use.
SeuratObject::SaveSeuratRds(object = screen_result, filename = "screened_result.rds")or in .h5ad format:
anndataR::write_h5ad(object= screen_result, path = "screened_result.h5ad", compression = "gzip")4.2 Continuous phenotype associated cell screening
Generally speaking, the process is the same as described in 5.1 Survival-associated cell screening. Here, only the preprocessing of continuous phenotypic data is introduced.
library(SigBridgeR)
library(zeallot)
library(Seurat)
setwd(here::here())
# * load the example data
c(mat_exam, bulk, pheno) %<-% LoadRefData(data_type = "continuous")
dim(mat_exam)
# [1] 33694 1093
dim(bulk)
# [1] 4106 289
bulk[1:6,1:6]
# TCGA-AZ-6599-01 TCGA-AA-3655-01 TCGA-A6-6137-01 TCGA-CK-4952-01 TCGA-A6-5657-01 TCGA-AD-6963-01
# HIF3A 2.3437 2.0858 6.0759 1.9506 5.4777 4.4634
# CAMK4 4.9331 2.3709 4.1387 1.1557 4.1746 3.2363
# RNF112 2.4817 2.4947 3.5941 2.3486 4.9185 1.4621
# SPN 5.6704 6.8577 8.0598 5.0049 7.6076 7.3960
# LRRTM1 1.6031 0.9465 1.9142 0.0000 3.2523 0.0000
# GRIN1 6.4944 4.3225 2.8073 7.3460 4.5000 3.1816
# * A named vector
head(pheno)
# TCGA-AZ-6599-01 TCGA-AA-3655-01 TCGA-A6-6137-01 TCGA-CK-4952-01 TCGA-A6-5657-01 TCGA-AD-6963-01
# 178 65 91 206 63 67 If your phenotype is a data.frame, try this to convert
it to a named vector:
pheno <- setNames(your_data.frame$continuous_numeric, your_data.frame$sample)4.3 Binarized phenotype associated cell screening
This process is also the same as described in 5.1 Survival-associated cell screening. Here, only the preprocessing of binary phenotypic data is introduced.
library(SigBridgeR)
library(zeallot)
library(Seurat)
setwd(here::here())
# * load the example data
c(mat_exam, bulk, pheno) %<-% LoadRefData(data_type = "binary")
dim(mat_exam)
#[1] 33694 1093
dim(bulk)
# [1] 4106 434
bulk[1:6,1:6]
# TCGA-CA-5256-01 TCGA-AZ-6599-01 TCGA-AA-3655-01 TCGA-A6-6137-01 TCGA-CK-4952-01 TCGA-A6-5657-01
# HIF3A 3.7172 2.3437 2.0858 6.0759 1.9506 5.4777
# CAMK4 3.0698 4.9331 2.3709 4.1387 1.1557 4.1746
# RNF112 1.3702 2.4817 2.4947 3.5941 2.3486 4.9185
# SPN 5.5207 5.6704 6.8577 8.0598 5.0049 7.6076
# LRRTM1 3.2408 1.6031 0.9465 1.9142 0.0000 3.2523
# GRIN1 3.0698 6.4944 4.3225 2.8073 7.3460 4.5000
# * A named vector
head(pheno)
# TCGA-CA-5256-01 TCGA-AZ-6599-01 TCGA-AA-3655-01 TCGA-A6-6137-01 TCGA-CK-4952-01 TCGA-A6-5657-01
# 1 1 1 1 1 1 If your phenotype is a data.frame, your binary variable
is stored in the “data” column, categorized as ‘Tumor’ and ‘Normal’, we
will assign ‘Tumor’ a value of 1 and ‘Normal’ a value of 0. In this way,
cells screened as Positive will be associated with
‘Tumor’. Try this to convert it to a named vector:
pheno <- mutate(
pheno,
data = dplyr::case_when(
data == "Tumor" ~ 1,
data == "Normal" ~ 0
)
)
pheno <- setNames(pheno$data, pheno$Sample)A PIPET use case
Previously, it was noted that PIPET cannot use patient survival data as the phenotype; here, we demonstrate using a binary phenotype instead.
pipet_result <- Screen(
matched_bulk = bulk,
sc_data = seurat,
phenotype = pheno,
phenotype_class = "binary",
screen_method = "PIPET",
label_type = "PIPET"
)
#ℹ [2025/12/26 06:18:28] Starting PIPET screen
#ℹ [2025/12/26 06:18:28] Creating marker genes from bulk data...
#ℹ [2025/12/26 06:18:28] Fitting model with limma
#✔ [2025/12/26 06:18:28] Created 2795 marker genes
#ℹ [2025/12/26 06:18:28] Running PIPET correlation analysis...
#ℹ [2025/12/26 06:18:28] The classification of markers is: group_0: 945 and group_1: 477
#ℹ [2025/12/26 06:18:28] Normalize count data with CPM and log1p
#ℹ [2025/12/26 06:18:29] Scale features with z-score normalization
#ℹ [2025/12/26 06:19:49] Organize the computed results
#✔ [2025/12/26 06:19:49] PIPET screening done.
table(pipet_result$scRNA_data$PIPET)
# Negative Neutral Positive
# 165 708 220 5. Troubleshooting
View Troubleshooting(https://github.com/WangX-Lab/SigBridgeR/wiki/Troubleshooting) for troubleshooting, especially for the environment setup.
Session information:
## R version 4.5.2 (2025-10-31)
## Platform: x86_64-pc-linux-gnu
## Running under: Ubuntu 24.04.3 LTS
##
## Matrix products: default
## BLAS: /usr/lib/x86_64-linux-gnu/openblas-pthread/libblas.so.3
## LAPACK: /usr/lib/x86_64-linux-gnu/openblas-pthread/libopenblasp-r0.3.26.so; LAPACK version 3.12.0
##
## locale:
## [1] LC_CTYPE=C.UTF-8 LC_NUMERIC=C LC_TIME=C.UTF-8
## [4] LC_COLLATE=C.UTF-8 LC_MONETARY=C.UTF-8 LC_MESSAGES=C.UTF-8
## [7] LC_PAPER=C.UTF-8 LC_NAME=C LC_ADDRESS=C
## [10] LC_TELEPHONE=C LC_MEASUREMENT=C.UTF-8 LC_IDENTIFICATION=C
##
## time zone: UTC
## tzcode source: system (glibc)
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## loaded via a namespace (and not attached):
## [1] digest_0.6.39 desc_1.4.3 R6_2.6.1 fastmap_1.2.0
## [5] xfun_0.56 cachem_1.1.0 knitr_1.51 htmltools_0.5.9
## [9] rmarkdown_2.30 lifecycle_1.0.5 cli_3.6.5 sass_0.4.10
## [13] pkgdown_2.2.0 textshaping_1.0.5 jquerylib_0.1.4 systemfonts_1.3.2
## [17] compiler_4.5.2 tools_4.5.2 ragg_1.5.1 bslib_0.10.0
## [21] evaluate_1.0.5 yaml_2.3.12 otel_0.2.0 jsonlite_2.0.0
## [25] rlang_1.1.7 fs_1.6.7 htmlwidgets_1.6.46. References
Sun D, Guan X, Moran AE, Wu LY, Qian DZ, Schedin P, et al. Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data. Nat Biotechnol. 2022 Apr;40(4):527–38.
Xie A, Wang H, Zhao J, Wang Z, Xu J, Xu Y. scPAS: single-cell phenotype-associated subpopulation identifier. Briefings in Bioinformatics. 2024 Nov 22;26(1):bbae655.
Zhang Q, Jin S, Zou X. scAB detects multiresolution cell states with clinical significance by integrating single-cell genomics and bulk sequencing data. Nucleic Acids Research. 2022 Nov 28;50(21):12112–30.
WangX-Lab/ScPP Internet. cited 2025 Aug 31. Available from: https://github.com/WangX-Lab/ScPP
Johnson TS, Yu CY, Huang Z, Xu S, Wang T, Dong C, et al. Diagnostic Evidence GAuge of Single cells (DEGAS): a flexible deep transfer learning framework for prioritizing cells in relation to disease. Genome Med. 2022 Feb 1;14(1):11.
Li J, Zhang H, Mu B, Zuo H, Zhou K. Identifying phenotype-associated subpopulations through LP_SGL. Briefings in Bioinformatics. 2023 Nov 22;25(1):bbad424.
Ruan X, Cheng Y, Ye Y, Wang Y, Chen X, Yang Y, et al. PIPET: predicting relevant subpopulations in single-cell data using phenotypic information from bulk data. Briefings in Bioinformatics. 2024 May 23;25(4):bbae260.